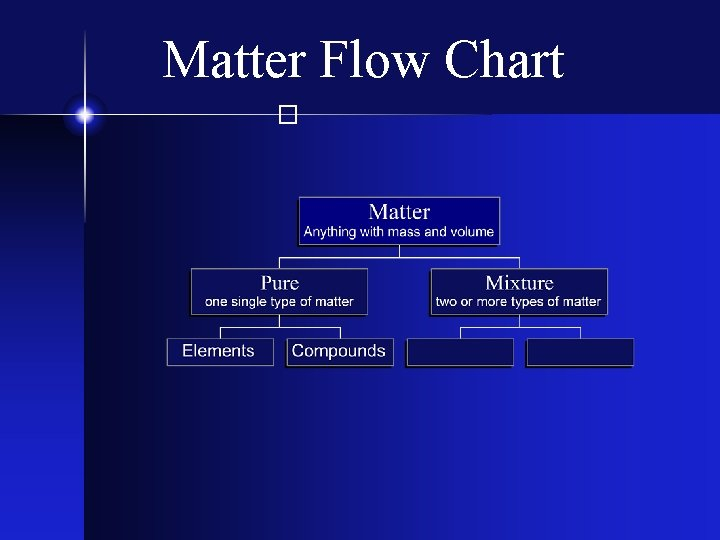
In the study of matter, we often encounter three primary states: solid, liquid, and gas. Each state has its own unique properties and characteristics. A flow chart can help visualize the transitions between these states.
Starting with a solid, the particles are closely packed together and have a fixed shape and volume. When heat is applied, the solid can melt into a liquid, where the particles are more spread out and can move past each other. Further heating can turn the liquid into a gas, where the particles are even more spread out and have no fixed shape or volume.
Flow Chart On Matter
Changes of State
Flow charts can also illustrate the changes of state that matter undergoes. For example, when a gas is cooled, it can condense into a liquid. This process is reversible, as heating the liquid can turn it back into a gas. Similarly, when a liquid is cooled further, it can solidify into a solid. These transitions can be represented in a flow chart to show the interconnectedness of the states of matter.
Flow charts are valuable tools in understanding the complexities of matter and its various states. By visually mapping out the transitions and changes of state, we can better grasp the fundamental principles that govern the behavior of matter.