Atoms are the building blocks of matter. They are the smallest units of a chemical element that retain the properties of that element. Atoms are made up of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus of the atom, while electrons orbit the nucleus in energy levels.
Molecules, on the other hand, are formed when two or more atoms bond together. These bonds can be covalent, ionic, or metallic, depending on the type of atoms involved. Molecules can be simple, like a water molecule (H2O), or complex, like DNA.
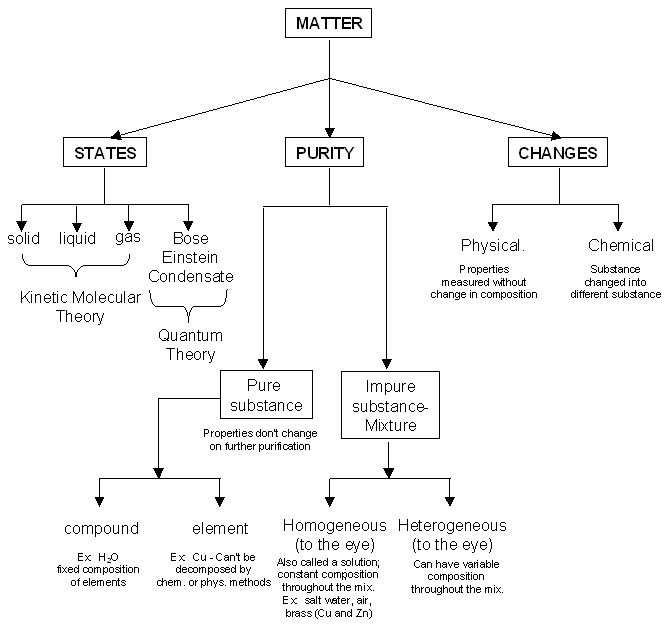
Atoms And Molecules Flow Chart
Understanding the Flow Chart
The atoms and molecules flow chart is a visual representation of how atoms combine to form molecules. It shows the different elements involved, as well as the types of bonds that are formed between them. The flow chart can help students understand the relationships between atoms and molecules, and how they interact in chemical reactions.
Each step in the flow chart represents a different stage in the process of forming molecules. For example, the first step may show the elements involved, the second step may show the types of bonds formed, and the final step may show the resulting molecule. By following the flow chart, students can see the progression from individual atoms to complex molecules.
Conclusion
Atoms and molecules are fundamental concepts in chemistry, and understanding how they interact is crucial for understanding the world around us. The atoms and molecules flow chart is a helpful tool for visualizing this process and can aid students in grasping these concepts more easily. By studying the flow chart, students can gain a deeper understanding of the relationships between atoms and molecules, and how they combine to create the substances we encounter every day.