When it comes to the decentralised procedure for the marketing authorization of medicinal products in the European Union, having a clear understanding of the flow chart is essential. This process involves the cooperation between multiple EU Member States in evaluating and approving a medicinal product for market access. The decentralised procedure flow chart outlines the steps and timelines involved in this collaborative process.
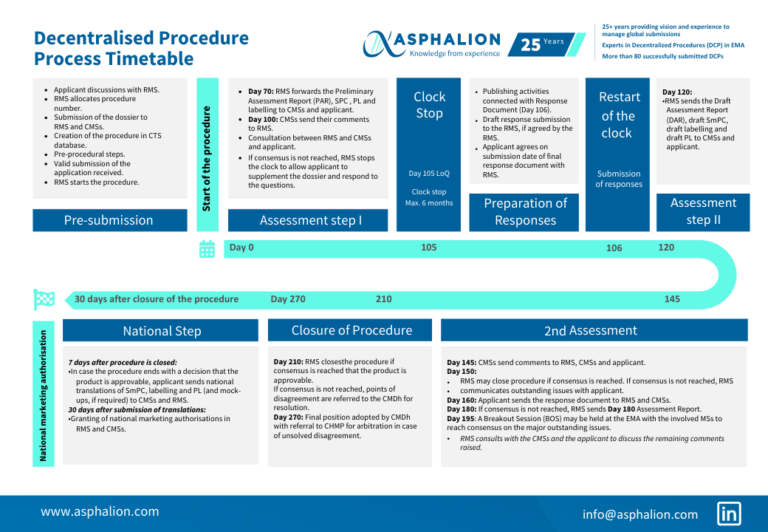
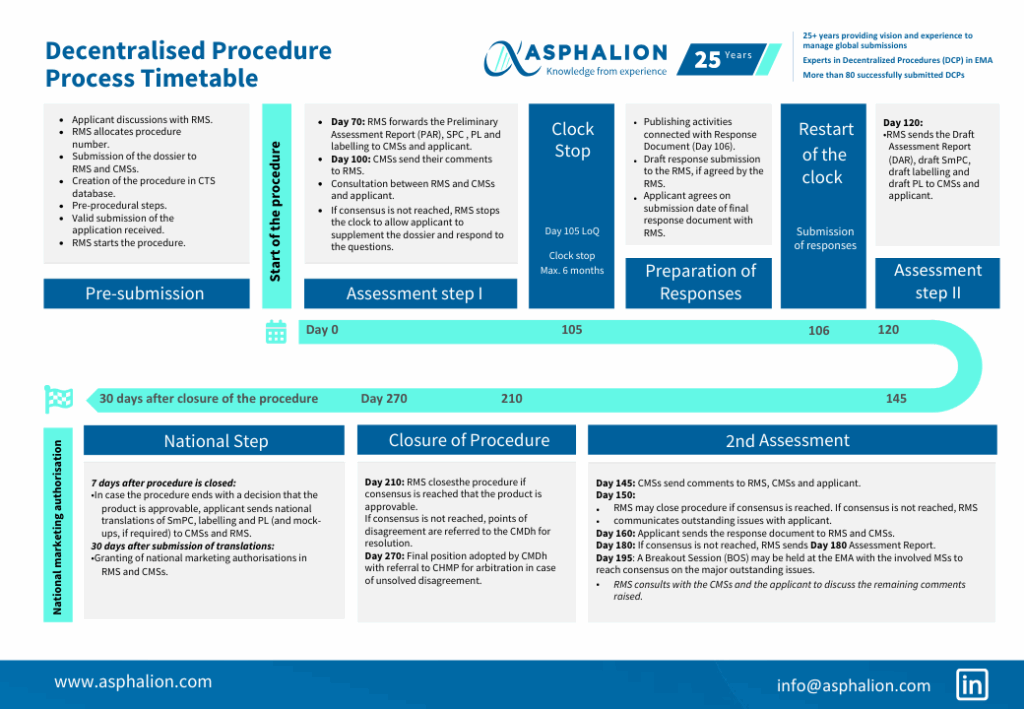
The decentralised procedure begins with the submission of a marketing authorization application to the Reference Member State (RMS). The RMS then distributes the application to Concerned Member States (CMS) within 15 days. The CMS have 90 days to evaluate the application and provide comments to the RMS. The RMS compiles all comments and prepares a draft assessment report within 120 days of the initial submission. This report is then circulated to all CMS for agreement.
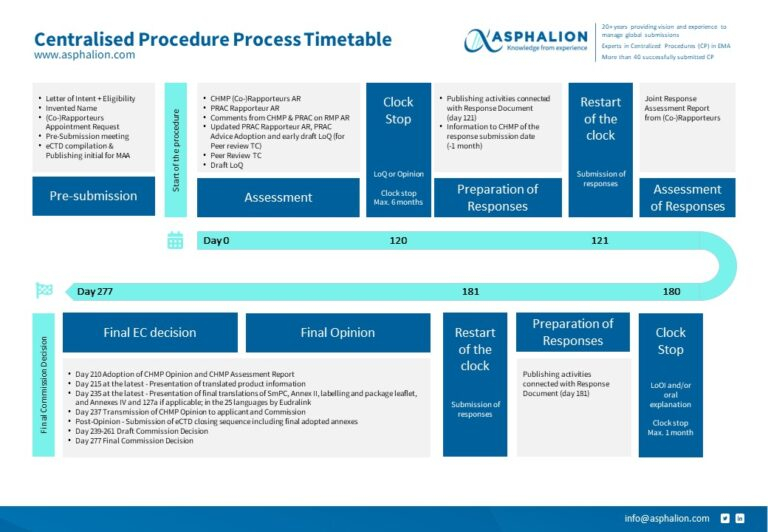
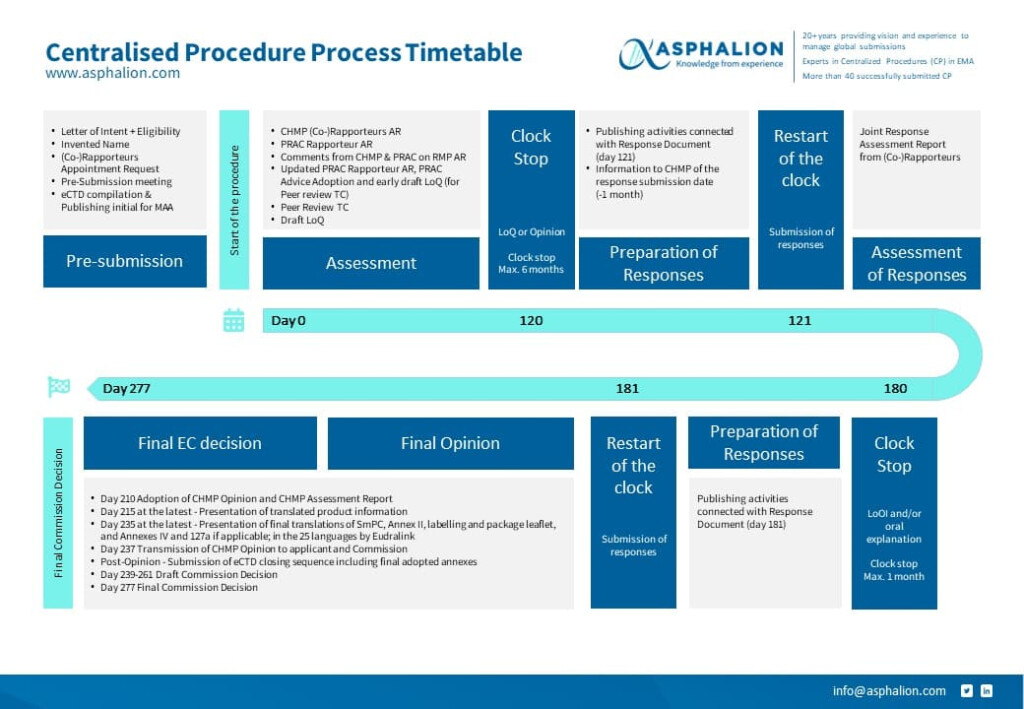
Decentralised Procedure Flow Chart
Benefits of Using the Decentralised Procedure Flow Chart
One of the main benefits of the decentralised procedure flow chart is the streamlined and harmonised approach to gaining marketing authorisation in multiple EU Member States. By following the flow chart, companies can navigate the complex regulatory landscape more efficiently and effectively. Additionally, the decentralised procedure allows for quicker access to the market for pharmaceutical products, benefiting both patients and manufacturers.
In conclusion, understanding the decentralised procedure flow chart is crucial for companies seeking marketing authorisation for medicinal products in the EU. By following the outlined steps and timelines, companies can navigate the regulatory process more smoothly and efficiently. This collaborative approach ensures that safe and effective medicinal products reach patients in a timely manner.
Download Decentralised Procedure Flow Chart
New CENTRALISED PROCEDURE PROCESS FLOW CHART
Decentralised Procedure Process Timetable Asphalion
New Process Timetable Of A Decentralised Procedure DCP In EMA
New Process Timetable Of A Decentralised Procedure DCP In EMA